
Sodium Electron Configuration With Full Orbital Diagram
In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure 6.24 depicts how these two trends in increasing energy relate.

Sodium Orbital Diagram
The periodic table By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior. Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns— groups —and rows— periods —that share certain properties.
Sodium Orbital Diagram
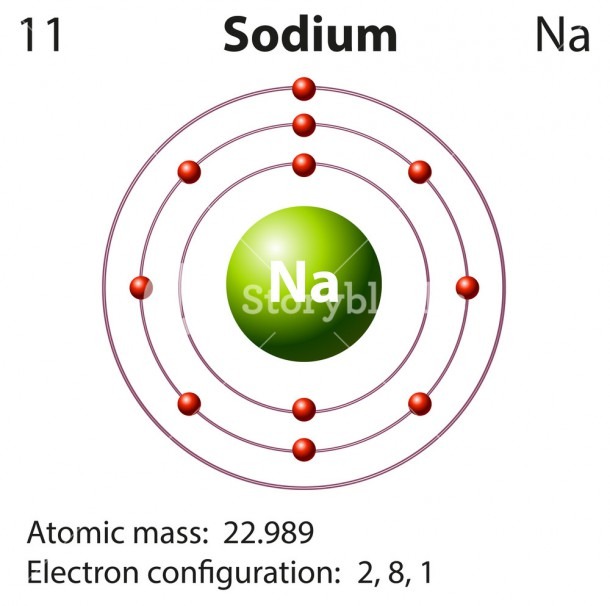
Sodium is the 11th element in the periodic table and its symbol is 'Na'. Sodium is a classified alkali metal element. In this article, I have discussed in detail how to easily write the complete electron configuration of sodium. What is the electron configuration for sodium? The total number of electrons in sodium is eleven.
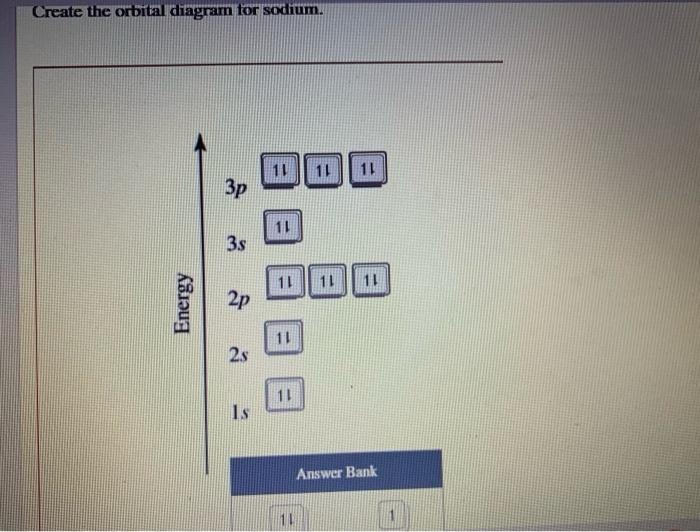
Solved Create the orbital diagram for sodium. 11 11 11 Зр 11
To write the orbital diagram for the Sodium atom (Na) first we need to write the electron configuration for just Na. To do that we need to find the number o.

Orbital Energy Diagram For Sodium
Sodium Electron Configuration (Na) with Orbital Diagram. Sodium Electron Configuration: The chemical element sodium has the symbol Na and atomic number 11. This is soft, reactive, silver + whitish metal. Electron configuration can define as the distribution of electrons of molecules or atoms in molecular or atomic orbits.

How to Write the Orbital Diagram for Sodium (Na)?
In this video we will write the electron configuration for Na+, the Sodium ion. We'll also look at why Sodium forms a 1+ ion and how the electron configurati.
Sodium Electron Configuration YouTube
Finally, the third shell would have a smaller circle with one arrow pointing up, representing the single electron in the 3s orbital. Sodium Electron Configuration Diagram. Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white metal that is highly reactive and can easily form compounds with other elements.
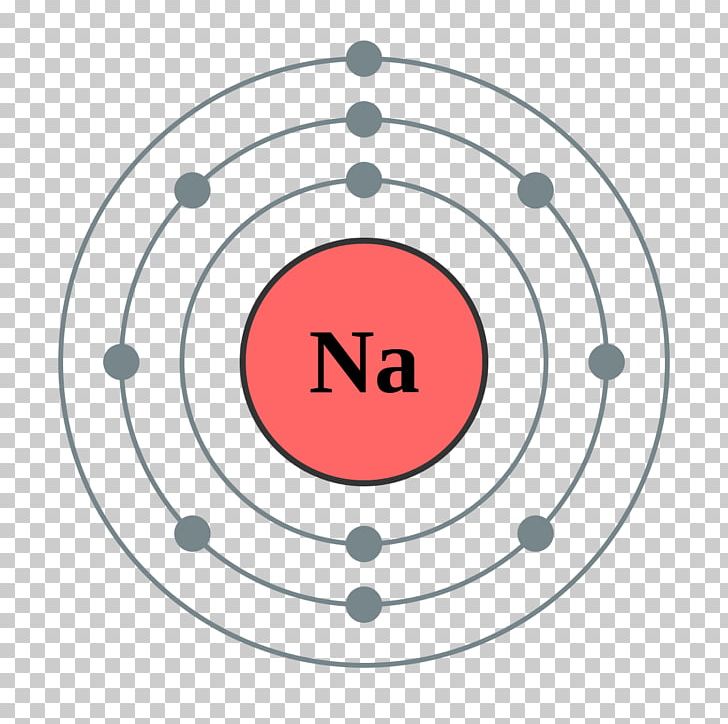
Electron Shell Sodium Electron Configuration Bohr Model PNG, Clipart
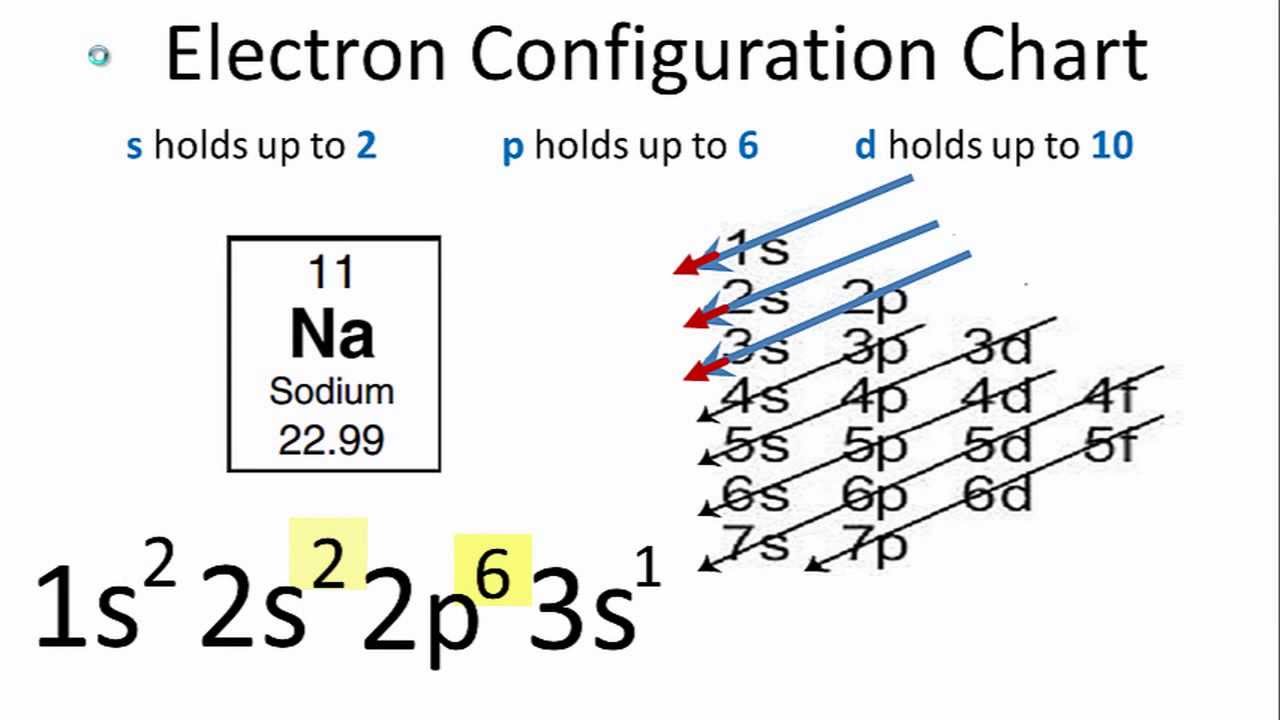
In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we'll put all 11 electrons in orbitals around the nucleus of the Sodium atom. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital.
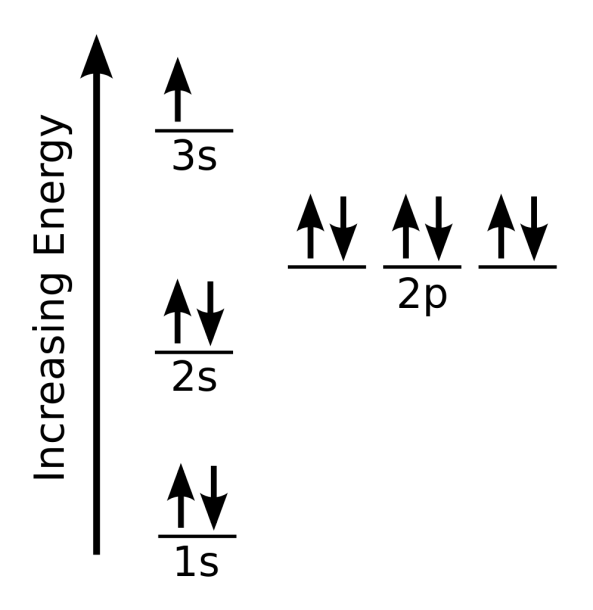
Sodium Orbital Diagram
Sodium orbital diagram The orbital diagram of sodium shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, and the 3s subshell has 1 electron. How to Write the Orbital Diagram for Sodium (Na) Watch on Contents Steps Find electrons Write electron configuration Draw orbital diagram Related

Introduction to Atoms
Sometimes, when p orbitals can't find another orbital has a similar symmetry with it, these p orbitals will remain as non-bonding orbitals. Figure 1.7.3 1.7. 3: This is the valence MO diagram of HF. The H1s orbital overlap with one of the F2p orbitals. The other two F2p orbitals remain as non-bonding orbitals.

orbital diagram of sodium atom. Brainly.in
The Na 3s 3 s orbital combines with Cl 3pz 3 p z to form the molecular orbitals labeled 4σ 4 σ and 4σ∗ 4 σ ∗ in Figure 5.3.3.1 5.3.3. 1. The 4σ 4 σ orbital is weakly bonding, but is very close in energy to the Cl 3pz 3 p z orbital, and is mostly Cl-like in character. Notice that all σ σ orbitals look very much like either s s or p p.

Sodium Orbital Diagram
0:00 / 2:00 Orbital Diagrams: Sodium Conrad Capule 69 subscribers Subscribe 12 1.8K views 2 years ago In this video, we determine how to draw the orbital diagram of sodium..more.more.
6.4 Electronic Structure of Atoms (Electron Configurations) Chemistry
Orbital Diagram. Nuclear. Radioactive: No: Isotopes. Symbol Mass Number Relative Atomic Mass Isotopic Composition; 18 Na: 18: 18.025970(50) 19 Na: 19: 19.013877(13). agriculture and photography. Sodium chloride (NaCl) is table salt. Liquid sodium is sometimes used to cool nuclear reactors. Sources Obtained by electrolysis of melted sodium.
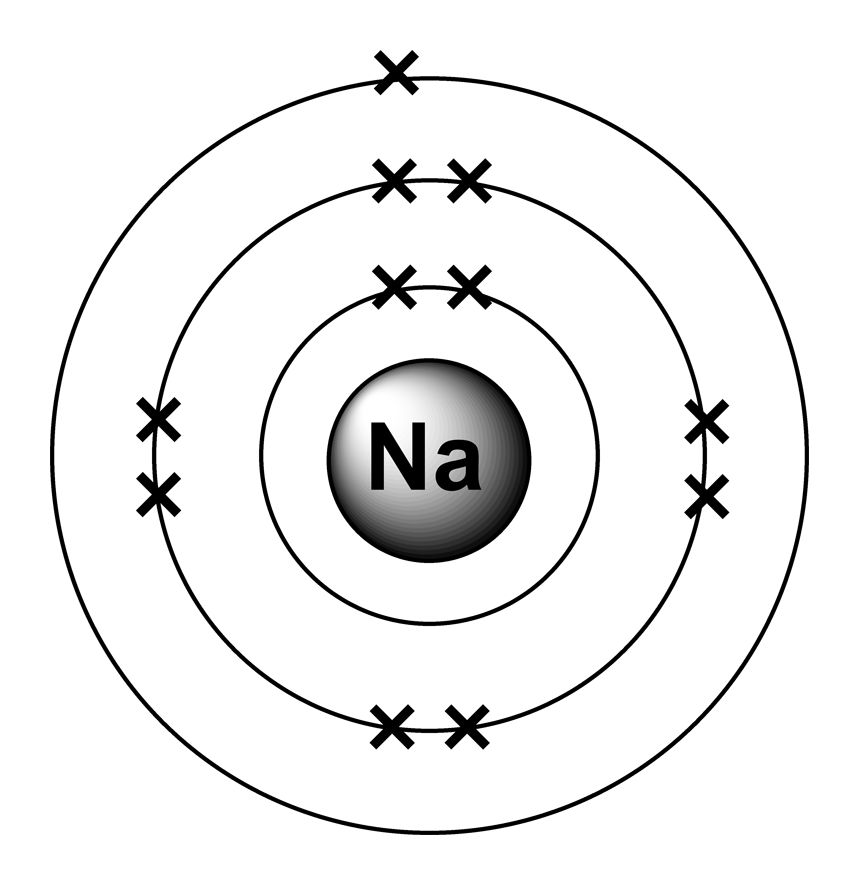
Sodium Table of Elements by Shrenil Sharma
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\).. An orbital diagram is the more visual way.

Orbital Energy Diagram For Sodium
Orbital Diagrams. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above.

Orbital Energy Diagram For Sodium
Referring to either Figure 2.6.3 2.6. 3 or 2.6.4 2.6. 4, we would expect to find the electron in the 1 s orbital. By convention, the ms = +1 2 m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.